| TOPIC | Medication Personal Care Worker Procedure | ||
| AREA | Service Delivery | TYPE | Procedure |
1. Purpose
Mobility is committed to ensuring all consumers receive their prescribed medications in a correct and safe manner in line with their right for self-determination and in accordance with current standards and legislation. Mobility is committed to the Quality Use of Medicines, to enhance the wellbeing of consumers.
2. Scope
This work Instruction applies to all Personal Care Workers who provide medication prompting to consumers.
3. Definitions
| Medication Prompting | The prompting of medication is reminder to a person of the time and asking if they have or are going to take their medicines.
The person is still in control of their medicines and may decide not to take them or to take them later.
Prompting can be useful when a person knows what medicines to take and how to take them but may simply forget the time. |
| Medication Errors | All medication errors or near misses are to be report to mobility. Errors could include, documentation error, incorrect dose, incorrect medication, incorrect route, incorrect time, given without order, missing medication, medication not given, medication not taken |
4. Procedure
4.1 All Personal Care Workers can supervise consumers’ medications if they have the following qualifications:
- Certificate III in Individual Support or higher or the equivalent and have completed a medication module in their certificate
- First Aid Certificate
- CPR certificate
- Medication Management training
4.2 The following conditions apply for medication prompting:
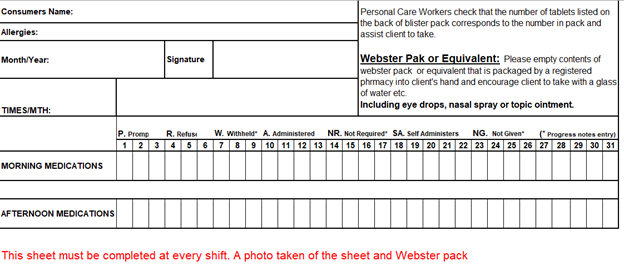
- There must be a prescription by a GP in place and all mediation must be in a webster pack or equivalent that is packaged by a registered pharmacy.
- The consumer has to be cognitive aware.
- PRN medication can only be supervised as per the prescription in place from the GP and the medication must be in the original packaging.
- All medications must be stored as per instructions.
4.3 The following can be applied or administered to the consumer:
- Non-prescribed topical treatments, except to perineal areas of body.
- Prescribed topical medication such as cortisone, rash treatments except the perinea! areas.
- Post-operative or .over the counter eye drops (refer to Eye Drops Work Instruction), Ear drops/ olive oil.
- Nasal and oral sprays.
- Topical medications applied to teeth.
- Tablets if stored in a Webster/ blister pack prepared by a registered Pharmacist.
All medications prompted except for eye drops, ointment and nasal sprays must be recorded on the Medication Tracking document see Appendix A.
4.4 What happens if there is a medication error, or you suspect the consumer has had an adverse reaction from their medication.
If it is an emergency, call 000 for an ambulance.
Or call the Poisons Information line on 131126 for all poisoning concerns or medication errors available 24/7 from anywhere in Australia.
In the event of a medication error:
- The Consumer’s GP is to be advised by the Care Manager or the consumers nominee/ Authorized
- Care Manager are to notify the Nominee/ Authorized
- All medication incidents must be investigated by the Clinical and Risk Specialist (CRS) or Carelynx RN as a priority within one working day or the next working day if occurring over the weekend or Public Holiday.
- The CRS is to be advised of all medication incidents and concerns.
- The CRS among other things will determine if any external authority reporting is required and progress as necessary.
5. Related Documents
Internal Documents
- Model of Care
- Infection Control Procedure
- Incident Management Procedure
- Eye Drops Work Instruction
- Medication Management Procedure
- Hand Hygiene Procedure
- Swallowing and Choking Procedure
- Personal Care Workers Procedure
External Documents
- Charter of Aged Care Rights (Clth)
- Australian Aged Care Act 1997 (Clth)
- Privacy Act 1988, Schedule 1, (Clth)
- Australian Privacy Principles 2014 (Clth)
- Controlled Substances Act 1984 (SA)
- Regulation 9 (Poisons) 2011
- Health Practitioner Regulation National Law (SA) Act 2010 and Regulation 2018
Version control:
| Version | Date | Change |
| 1.0 | September 2021 | New |
APPENDIX A Medication Tracking Sheet